 |  |  How does the horseshoe crab protect the public health? How does the horseshoe crab protect the public health?
The horseshoe crab plays a vital, if little-known, role in the life of anyone who has received an injectable medication. An extract of the horseshoe crab's blood is used by the pharmaceutical and medical device industries to ensure that their products, e.g., intravenous drugs, vaccines, and medical devices, are free of bacterial contamination. No other test works as easily or reliably for this purpose. Read below for more detail. Why are we concerned about bacterial contamination of pharmaceutical products? Bacteria are everywhere-from our intestinal tract, to soils, rivers, and oceans. For the most part, bacteria are beneficial, acting to degrade organic waste and recycle nutrients back into the food chain. Sometimes, however, bacteria cause disease. We are all familiar with many specific bacterial diseases such as Salmonella food poisoning or more serious ones such as Cholera and Tetanus. Bacteria that cause these diseases are referred to as pathogens and usually require an animal host for multiplication or transmission even though they may persist in a soil or aquatic environment for long periods of time. Other bacteria, generally considered non-pathogenic, can cause disease if they enter parts of our body that are usually bacteria-free, such as the bloodstream. In this case, even the ordinarily benign gut bacterium E. coli can cause sepsis and death. Therefore, the pharmaceutical industry takes great care in producing drugs, vaccines, and medical devices (items that deliver drugs or are implanted) that are sterile-free of living microorganisms. Unfortunately, certain bacterial components can, in and of themselves, be toxic. Thus, pharmaceutical manufacturers not only need to be sure their products are sterile but also non-toxic, i.e., contain no bacterial components left from pre-sterilization bacterial contamination. 
Illustration credit Charles River Endosafe, SC |
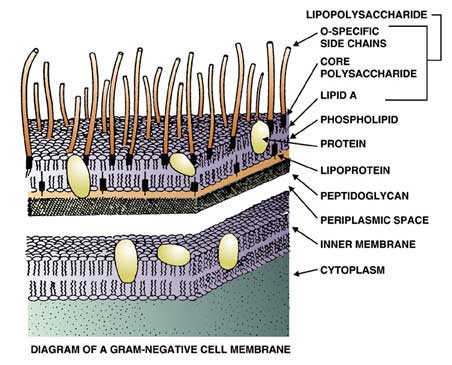
The bacterial toxin of greatest concern is termed endotoxin, and it is able to withstand steam sterilization. Endotoxin occurs as part of the cell structure of a large class of bacteria that includes both pathogens and non-pathogens. This class of bacteria is known as Gram-negative, for their characteristic of being easily decolorized during the Gram staining procedure. Surprisingly, it is the non-pathogenic members of the Gram-negative group, those that love aquatic environments, which cause the most problems for the pharmaceutical industry. Over fifty years ago it was recognized that some sterile solutions, when injected into humans or rabbits, caused a fever or pyrogenic response. Scientists soon learned that these so-called "injection fevers" were caused by endotoxin (a potent pyrogen) left over from bacterial components that remained in the injected solutions after sterilization. Fortunately, it was also found that solutions could be screened for pyrogens by injecting small amounts of the batch into rabbits. If the rabbit exhibited a fever, the solution was deemed pyrogenic and was rejected. The rabbit or pyrogen test, along with a sterility test, became the two most important tools of the pharmaceutical industry. The Pyrogen Test employing rabbits is still in limited use, although as you will see below, an endotoxin test using an extract from the blood cells of the horseshoe crab is the predominant pyrogen test today. How was the horseshoe crab test discovered? In the 1960's, Dr. Frederik Bang, a Johns Hopkins researcher working at the Marine Biological Laboratory in Woods Hole, Massachusetts, found that when common marine bacteria were injected into the bloodstream of the North American horseshoe crab, Limulus polyphemus, massive clotting occurred. Later, with the collaboration of Dr. Jack Levin, the MBL team showed that the clotting was due to endotoxin, a component of the marine bacteria originally used by Dr. Bang. In addition, these investigators were able to localize the clotting phenomenon to the blood cells, amebocytes, of the horseshoe crab, and, more importantly, to demonstrate the clotting reaction in a test tube. The cell-free reagent that resulted was named Limulus amebocyte lysate, or LAL. The name LAL is extremely descriptive: Limulus is the generic name of the horseshoe crab, amebocyte is the blood cell that contains the active components of the reagent, and lysate describes the original process used by Levin and Bang to obtain these components. In Levin and Bang's process, amebocytes, after being separated from the blue-colored plasma (hemolymph), were suspended in distilled water where they lysed (ruptured) due to the high concentration of salt contained in the amobocytes versus the absence of salt in the distilled water. Surprisingly, this same process with some minor modifications is still used today to produce LAL. How does the horseshoe crab protect itself from disease? One may wonder why the horseshoe crab is sensitive to endotoxin and, furthermore, how does the crab benefit from this phenomenon? As we know, seawater is a virtual "bacterial soup". Typical near-shore areas that form the prime habitat of the horseshoe crab can easily contain over one billion Gram-negative bacteria per milliliter of seawater. Thus, the horseshoe crab is constantly threatened with infection. Unlike mammals, including humans, the horseshoe crab lacks an immune system; it cannot develop antibodies to fight infection. However, the horseshoe crab does contain a number of compounds that will bind to and inactivate bacteria, fungi, and viruses. The components of LAL are part of this primitive "immune" system. The components in LAL, for example, not only bind and inactivate bacterial endotoxin, but the clot formed as a result of activation by endotoxin provides wound control by preventing bleeding and forming a physical barrier against additional bacterial entry and infection. It is one of the marvels of evolution that the horseshoe crab uses endotoxin as a signal for wound occurrence and as an extremely effective defense against infection. How are the horseshoe crabs collected? Are they harmed? In shallow water, horseshoe crabs are collected by hand from a small boat using a clam rake, and the animals are not injured during this process. In deeper water, a dredge is used, and in this case, some horseshoe crabs do get injured. Injured crabs are released immediately and most will survive. It is quite common to find crabs with "scars" of old injuries that have healed. Once the crabs are caught, they are transported to the laboratory from the fishing pier by truck. Sometimes a refrigerated truck is used, but as long as the animals are kept cool and dark during transport, they exhibit no adverse affects. During the bleeding process, up to 30% of the animal's blood is removed. Research has shown that once returned to the water, the horseshoe crab's blood volume rebounds in about a week. It takes longer for the crab's blood cell count to return to normal, about two to three months. Theoretically, crabs can be bled several times a year, but LAL manufacturers bleed them only once per year. The Associates of Cape Cod and other LAL manufacturers have studied horseshoe crab mortality following the bleeding procedure and have found it to be quite low, less than 3% when compared to controls handled similarly but not bled. There are no records of a horseshoe crab dying during the bleeding process itself. Other studies conducted by government agencies and universities indicate a mortality of 10-15%. However, the horseshoe crabs in these studies were not handled as carefully as those collected by the LAL industry. Studies done by the Associates of Cape Cod show that not only do the crabs survive one bleeding, but that they can be captured year after year to donate their life-saving blood-much like human blood donors. In addition, their studies indicate that crabs, which are bled and returned to their spawning area, will continue their breeding activity without any ill effect. The companies that produce LAL go to great lengths to ensure that the animals used in the making this valuable, life-saving test are handled with care and respect. They recognize that a stable horseshoe crab population is vitally important not only to the biomedical community, but also to the survival of millions of shorebirds, sea turtles, and other marine creatures that have a symbiotic relationship with this remarkable creature. These companies will continue to support sound, scientifically-based conservation measures that will ensure a sustainable population for the future.  Product Product
 Bleeding Bleeding
|  Bottling Bottling |  Collecting Collecting | Photographs provided by Associates of Cape Cod |
How was LAL commercialized? In the 1970's, Dr. Stanley Watson, a scientist at the Woods Hole Oceanographic Institution located across the pond from the MBL, began using the LAL reagent in his research with marine bacteria. Since no commercial reagent existed, Dr. Watson set up production for his own use. However, word quickly spread that Dr. Watson was sharing excess reagent, not only with other scientists interested in bacterial endotoxin, but also with pharmaceutical companies interested in using LAL as an in-process control for endotoxin contamination. When the demand for LAL outpaced supply, Dr. Watson decided to set up a small company, Associates of Cape Cod, Inc. To reward his efforts, Dr. Watson's company was granted the first Food and Drug Administration license to manufacture and sell LAL. As the LAL became accepted as a replacement for the rabbit pyrogen test and global demand for the reagent grew, other US companies and one Japanese company were licensed. Today LAL is made in the US, Japan, and China. Commercial LAL is produced in a manner nearly identical to the procedure described by Levin and Bang, albeit on a larger scale. While Levin and Bang used their lysate directly in their experiments, commercial LAL is freeze-dried to give it a longer shelf life and allow for easier shipping. How is an LAL test performed? To use the commercial product, a laboratory reconstitutes the vial of freeze-dried LAL with endotoxin-free water. An equal amount of reconstituted LAL, usually 0.1 ml, is then added to the sample solution in a small, glass, endotoxin-free test tube. The mixture is then incubated at 37C for one hour. At the end of this time, the mixture is examined for gel formation by gently inverting the tube. If sufficient endotoxin was present in the sample, a firm gel, one that can withstand inversion of the tube, is formed. Knowing the sensitivity of the LAL then allows the investigator to determine the quantity of endotoxin in the sample. If the sample is found to contain an amount that exceeds the limit set by the FDA, the sample fails and the lot of pharmaceutical product must be rejected. The US FDA currently requires the LAL test to be performed on all human and animal injectables as well as medical devices used to deliver these injectables. In addition, many implantable devices and artificial kidneys used for renal dialysis also require an LAL test. Are there other uses for LAL? Since LAL detects endotoxin, a component of Gram-negative bacteria, the test can also be used to detect the presence of these bacteria. However, there are two major drawbacks: 1. LAL cannot discriminate between living and dead bacteria, and
2. LAL cannot differentiate species of bacteria-endotoxin, which cause a similar reaction with LAL.
Even with these drawbacks, the LAL test has been used to rapidly diagnose urinary tract infections and spinal meningitis. In these cases, the presence of endotoxin is almost always indicative of living bacteria, i.e., an infection, and the types of bacteria causing these infections are few and quite similar. The LAL test has also been used to assess food spoilage (fish, milk, ground beef), air and water quality, and (in experiments) to determine the ability of new drugs to neutralize the toxic effects of endotoxin. Are there other compounds in the horseshoe crab that are of biomedical interest? Besides LAL, a number of reagents and medically useful compounds have been discovered in the blood of the horseshoe crab. These include: - A new test for fungal infections (G-Test) which is already in use in Japan and is expected to be licensed in the US next year
- An endotoxin-neutralizing protein which has potential as an antibiotic as well as an alternative endotoxin assay. This protein, ENP, can be made synthetically, which would eliminate the use of live horseshoe crabs for the LAL reagent.
- A number of other proteins that show anti-viral and anti-cancer activity.
Written for ERDG by: Thomas J. Novitsky, Ph.D.
Edited by: Lisa Smith Infectious diseases are the third cause of death in the United States and are the leading cause worldwide. The LAL is a major tool in the development of new antibiotics and vaccines. LAL Manufacturers Associates of Cape Cod, MA
Cambrex Corporation
Charles River Endosafe, SC
Horseshoe Crabs and Vision It was in 1926 that H. Keffer Hartline began to study electrical impulses from the optic nerve of horseshoe crab eyes. From these studies, some important principles about the function of human eyes were discovered. As a result, Dr. Ragnar Granit of Sweden and Americans H. Keffer Hartline and George Wald were awarded the 1967 Nobel Prize in Medicine. Chitin The chitin from horseshoe crabs is used in the manufacturing of chitin-coated filament for suturing and chitin-coated wound dressing for burn victims (Hall, 1992). Since the mid-1950s, medical researchers have known that chitin-coated suture material reduces healing time by 35 to 50 percent. Pharmaceutical, Biomedical and LAL Industry Sponsors of Horseshoe Crab Conservation |












 Product
Product  Bleeding
Bleeding  Bottling
Bottling Collecting
Collecting